Rheumatology basics
Most rheumatic disease can be grouped into 10 major categories:
Systemic connective tissue disease
Vasculitides
Seronegative spondyloarthropathies
Arthritis associated with infectious agents
Rheumatic disorders with metabolic, endocrine, and hematologic disease
Bone and cartilage disorders
Hereditary, congenital and inborn errors of metabolism associated with rheumatic syndromes
Nonarticular and regional MSK disorders
Neoplasms and tumorlike lesions
Miscellaneous rheumatic disorders
The terms collagen-vascular disease and connective tissue disease are use synonymously, although the purist would say that the inherited collagen disorders are the only true diffuse collagen diseases.
Anatomy and physiology of the musculoskeletal system
Collagen
Collagen is the most abundant protein in the human body, accounting for 20%-30% of the total body mass. There are at least 14 different types of collagen. The unique properties and organization of each collagen type enables that specific collagen to contribute to the function of the tissue which it is the principal structural component. Collagen type I-V are the five most abundant collagen.
FACIT = Fibril-associated collagens with interrupted triple helices
Type | Class | Tissues |
|---|---|---|
I | Interstitial | Bone, tendon, joint capsule and synovium, skin |
II | Interstitial | Hyaline cartilage, vitreous of eye |
III | Interstitial | Blood vessels, intestine |
IV | Basement membrane | Lamina densa of basement membrane |
V | Interstitial | Same as type I collagen |
VI | Nonfibrillar | Aortic intima, skin, kidney, muscle |
VII | Nonfibrillar | Amnion, dermoepidermal anchoring fibrils |
VIII | Short-chain | Endothelial cells, Descment's membrane |
IX | FACIT | Same as type II collagen, cornea |
X | Short-chain | Growth plate cartilage |
XI | Interstitial | Hyaline cartilage |
XII | FACIT | Same as type I collagen |
XIII | Nonfibrillar | Endothelial cells |
XIV | FACIT | Skin, tendon |
The definitive structural features of all collagen molecules is the triple helix. This unique conformation is due to three polypeptide chains (alpha-chains) twisted all around each other into a right handed major helix.
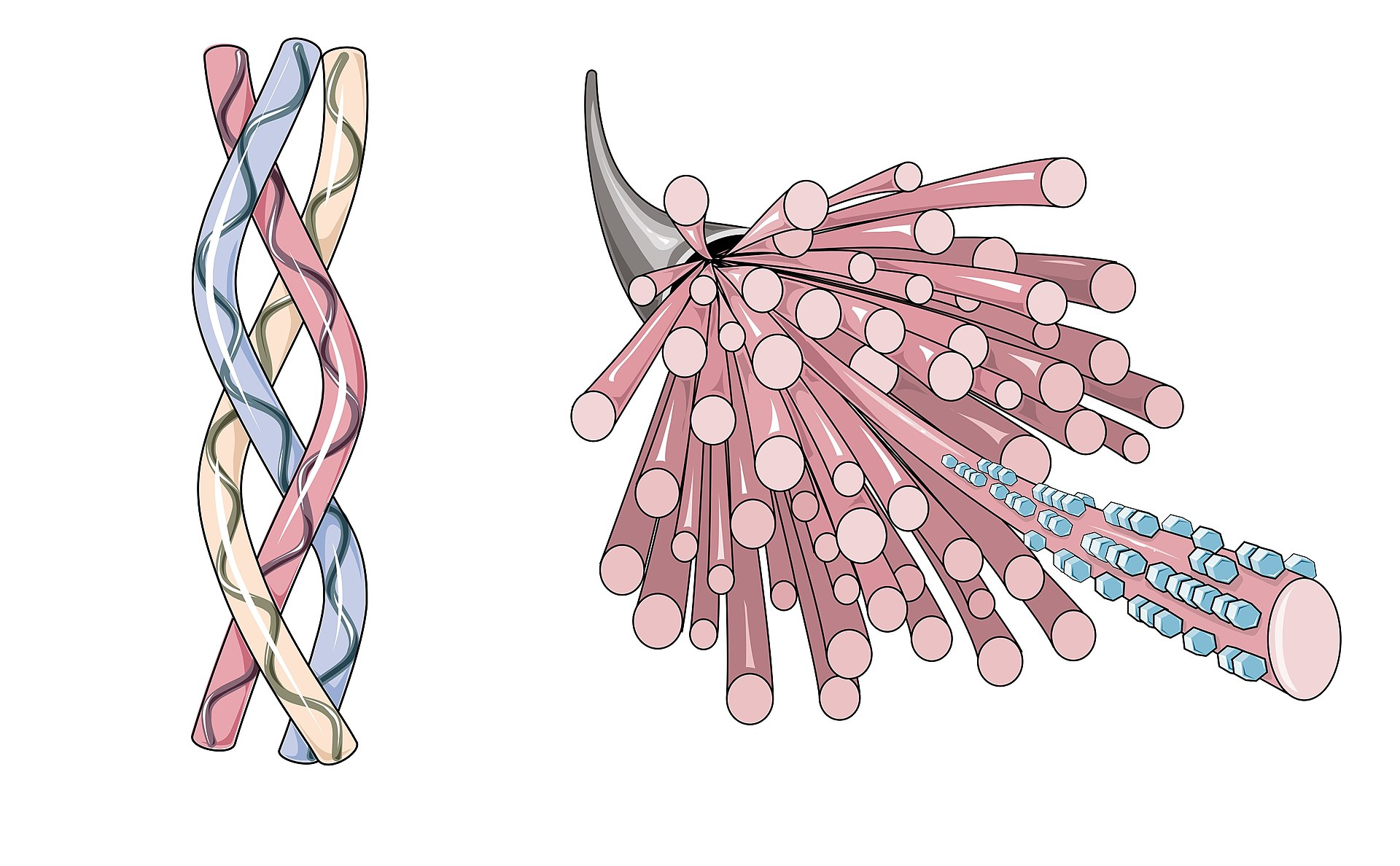
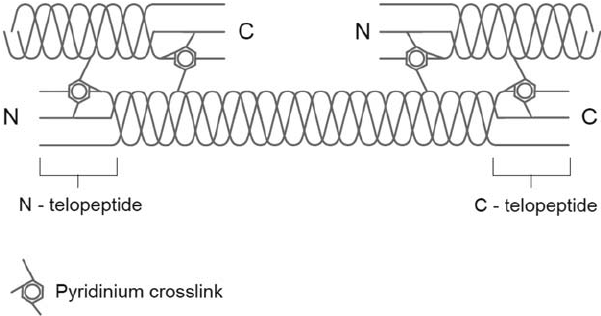
Extending from the amino and carboxyl terminal ends of both helical domains of the alpha-chains are nonhelical components called telopeptides. In the major interstitial collagens, the helical domains are continuous, whereas in the other collagen classes (nonfibrillar, short-chain and FACIT), the helical domains may be interrupted to 1 to 12 nonhelical segments.
The primary structure of the helical domain of the alpha-chain is characterized by the repeating triplet X-Y-Gly, and X and Y can be any amino acid but are most frequently:
Proline
Hydroxyproline
Approximately 25% of the residues in the triple helical domains consist of proline and hydroxyproline. Hydroxylysine is also commonly found. In the most abundant interstitial collagens (type I and II), the triple helical region contains about 100 residues (X-Y-Gly).
The interstitial collagens are the most abundant collagen class; they form the extracellular fabric of the major connective tissues. Fibril-associated collagens with interrupted triple helices (FACIT) are associated with the interstitial fibrillar collagens and occur in the same tissues.
Interstitial/Fibrillar collagens
Type I (1)
Type II (2)
Type III (3)
Type V (5)
Type XI (11)
Fibril-associated collagens with interrupted triple helices
Type IX (9)
Type XII (12)
Type XIV (14)
Specialized structures/function
Type IV (4) - Basement membrane collagen
Type VI (6), VII (7), XIII (13) - Nonfibrillar collagens
Types VIII (8), X (10) - Short chain collagens
Synthesis of collagen
The synthesis of collagen is important due to the pathophysiology of collagen disease and their presentations. There are 8 main steps, which begins with the encoding genes.
There are 20 distinct genes coding the various chains - The DNA is transcribed to form a precursor mRNA, which is processed to functional mRNA by excising and splicing which removes mRNA coded by introns. The processed mRNAs leave the nucleus and transported to the polyribosome apparatus in the rough endoplasmic reticulum for translation into polypeptide chains
The polypeptide chains are hydroxylated by prolyl hydroxylase and lysine hydroxylase. These enzymes require O2, Fe2+, alpha-ketoglutarate, and ascorbic acid as cofactors. Hydroxyproline is critical to the stable formation of the triple helix. A decrease in hydroxyproline content as seen in scurvy (ascorbic acid deficiency) results in unstable molecules that lose their structures and are broken down by proteases
Glycosylation of hydroxylysine residues, which is important for secretion of procollagen monomers (molecules)
Formation of interchain disulfide links, followed by procollagen triple-helix formation
Secretion of procollagen into the extracellular space
Proteolysis by procollagen peptidase of amino and carboxyl terminal telopeptides, resulting in conversion of procollagen to collagen
Assembly of collagen monomers into fibrils (microfibrils) by quarter-stagger shift, followed by crosslinking of fibrils
End-to-end and lateral aggregation of fibrils to form collagen fiber
Each collagen molecule is 300nm in length and 1.5nm in width and has five charged regions 68nm apart. The charged regions align in a straight line when the fibrils are formed, even though the individual molecules themselves are staggered a quarter of their lengths in relation to each other. One can easily see that there are multiple steps where defects in collagen biosynthesis could result in abnormalities leading to disease.
Collagen degradation
The most important collagenolytic enzymes responsible for cleavage of type I collagen belong to the matrix metalloproteinase group (MMP). The enzyme MMP1 is secreted in latent form, and when activated, cleaves the collagen molecule at a single specific site 75% from the amino terminal end (between residues 775-776 of alpha-1 (I) chain). Gelatinases and stromelysin degrade the unfolded fragments. Both alpha-2-macroglobulin and tissue inhibitor of metalloproteases (TIMP) are capable of inhibiting collagenase activity. It is likely that other collagen types have type-specific collagenases capable of degrading them. Serum procollagen peptides, urinary hydroxyproline, and urinary pyridinoline/deoxypyridinoline cross-links are used as measures of collagen turnover.